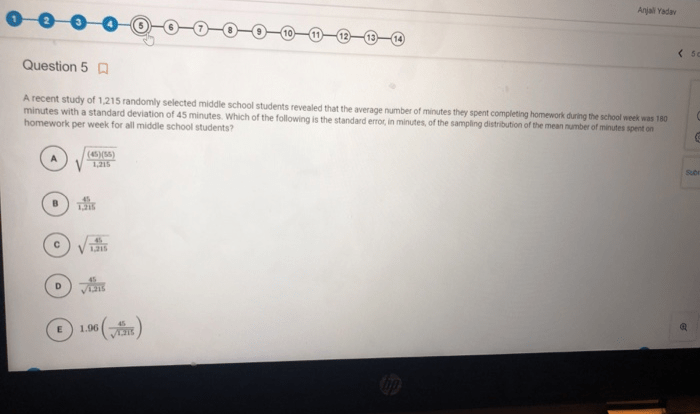
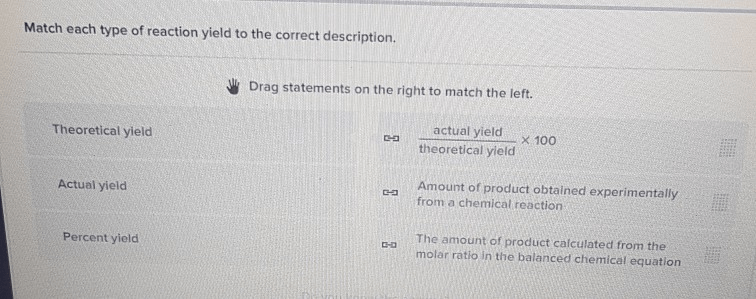
Match each type of reaction yield to the correct description. – Match Reaction Yield Types to Their Descriptions explores the fundamental concepts and practical applications of reaction yield in chemical reactions. This topic holds immense significance in various fields, including chemical synthesis and pharmaceutical manufacturing, where optimizing reaction yield is crucial for efficiency and cost-effectiveness.
Delving into the different types of reaction yields, we will examine theoretical yield, actual yield, and percent yield, highlighting their significance and the factors that influence them. Understanding these concepts is essential for chemists and researchers seeking to maximize reaction outcomes and advance scientific discoveries.
Types of Reaction Yields
Reaction yield is a crucial concept in chemistry that measures the efficiency of a chemical reaction. It is expressed as the ratio of the actual amount of product obtained to the theoretical amount of product that could be produced based on the stoichiometry of the reaction.
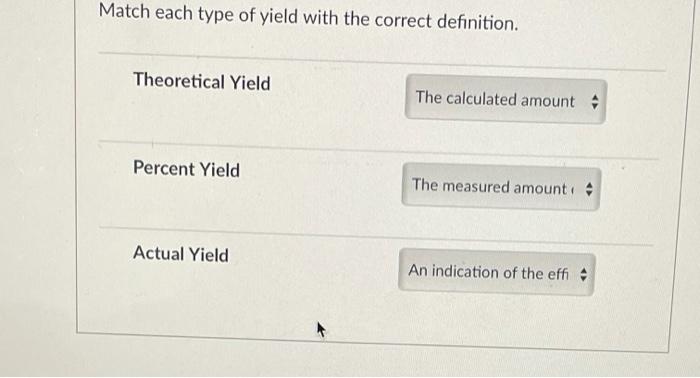
Theoretical Yield
The theoretical yield is the maximum amount of product that can be formed based on the limiting reactant and the stoichiometry of the reaction. It is calculated using the mole ratios of the reactants and products.
Actual Yield
The actual yield is the amount of product that is actually obtained in the reaction. It is typically less than the theoretical yield due to factors such as incomplete reactions, side reactions, and losses during the isolation and purification of the product.
Percent Yield
The percent yield is a measure of the efficiency of a reaction and is calculated by dividing the actual yield by the theoretical yield and multiplying by 100.
Factors Affecting Reaction Yield
Several factors can influence the yield of a reaction, including:
Temperature, Match each type of reaction yield to the correct description.
Increasing the temperature generally increases the reaction rate and the yield of the product. This is because higher temperatures provide more energy for the reactants to overcome the activation energy barrier.
Concentration
Increasing the concentration of the reactants increases the likelihood of collisions between them, leading to a higher reaction rate and yield.
Catalysts
Catalysts are substances that increase the reaction rate without being consumed in the reaction. They provide an alternative pathway for the reaction to occur, lowering the activation energy and increasing the yield.
Methods to Improve Reaction Yield
There are several techniques that can be employed to optimize reaction conditions and improve the yield of a reaction:
Using Catalysts
As mentioned earlier, catalysts can significantly increase the reaction rate and yield. Choosing the appropriate catalyst for a specific reaction can lead to substantial improvements.
Adjusting Temperature
Optimizing the reaction temperature can also enhance the yield. Finding the optimal temperature range where the reaction proceeds efficiently while minimizing side reactions is crucial.
Applications of Reaction Yield
Reaction yield plays a vital role in various fields:
Chemical Synthesis
In chemical synthesis, maximizing the yield of desired products is essential for efficient and cost-effective production of chemicals, pharmaceuticals, and other materials.
Pharmaceutical Manufacturing
In the pharmaceutical industry, high reaction yields are crucial to ensure the production of pure and effective drugs while minimizing waste and production costs.
Q&A: Match Each Type Of Reaction Yield To The Correct Description.
What is the significance of reaction yield in chemical synthesis?
Reaction yield is crucial in chemical synthesis as it determines the efficiency and cost-effectiveness of the process. A higher reaction yield indicates a greater conversion of reactants into the desired product, minimizing waste and maximizing resource utilization.
How can reaction yield be improved?
Reaction yield can be improved by optimizing reaction conditions, such as temperature, concentration, and the use of catalysts. Additionally, employing techniques like reflux, distillation, and extraction can further enhance yield.