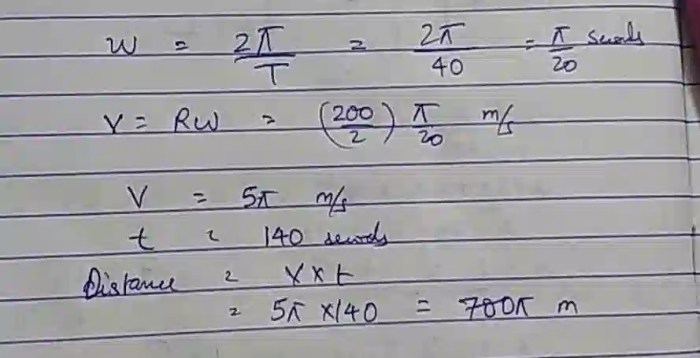Unveiling the intricacies of electrostatics, the Coulombic attraction POGIL answer key unlocks a world of charged particles and their captivating interactions. Delve into the depths of this key, where the forces that govern the behavior of ions and molecules come to life, shaping the properties of matter and driving countless applications.
Within the realm of Coulombic attraction, we uncover the mathematical formula that quantifies the strength of these electrostatic forces, revealing the dance between distance, charge, and the fundamental constant known as the permittivity of free space. As we explore the factors that modulate Coulombic attraction, we gain insights into the behavior of ionic and covalent compounds, paving the way for understanding their unique characteristics.
Coulombic Attraction: Definition and Overview: Coulombic Attraction Pogil Answer Key
Coulombic attraction is a fundamental force that describes the interaction between electrically charged particles. It is named after the French physicist Charles-Augustin de Coulomb, who first mathematically described the force in 1785. Coulombic attraction is a long-range force, meaning that it can act over large distances, and it is one of the four fundamental forces of nature, along with gravity, the weak force, and the strong force.
The strength of the Coulombic attraction between two charged particles is directly proportional to the magnitude of the charges and inversely proportional to the square of the distance between them. The mathematical formula for Coulombic attraction is:
F = k
- (q1
- q2) / r^2
where:
- F is the force of attraction
- k is Coulomb’s constant (8.98755 × 10^9 N⋅m^2/C^2)
- q1 and q2 are the magnitudes of the charges
- r is the distance between the charges
The sign of the force is positive if the charges are opposite (i.e., one positive and one negative) and negative if the charges are the same (i.e., both positive or both negative).
Q&A
What is Coulomb’s law?
Coulomb’s law provides the mathematical formula for calculating the force of attraction or repulsion between two charged particles.
How does Coulombic attraction affect the properties of materials?
Coulombic attraction influences properties such as melting point and electrical conductivity by determining the strength of the interactions between ions or molecules.
What role does Coulombic attraction play in chemical bonding?
Coulombic attraction is a key factor in the formation and stability of ionic bonds, where oppositely charged ions are held together by electrostatic forces.